Potential to be the leading Treatment for Oral Pain


Our lead indication is for pain caused by Oral Mucositis and BupiZenge™ contains the effective non-opioid long acting reliever bupivacaine. By applying bupivacaine in a lozenge we effectively relieves pain locally in the mouth and throat which solves this unmet medical need.
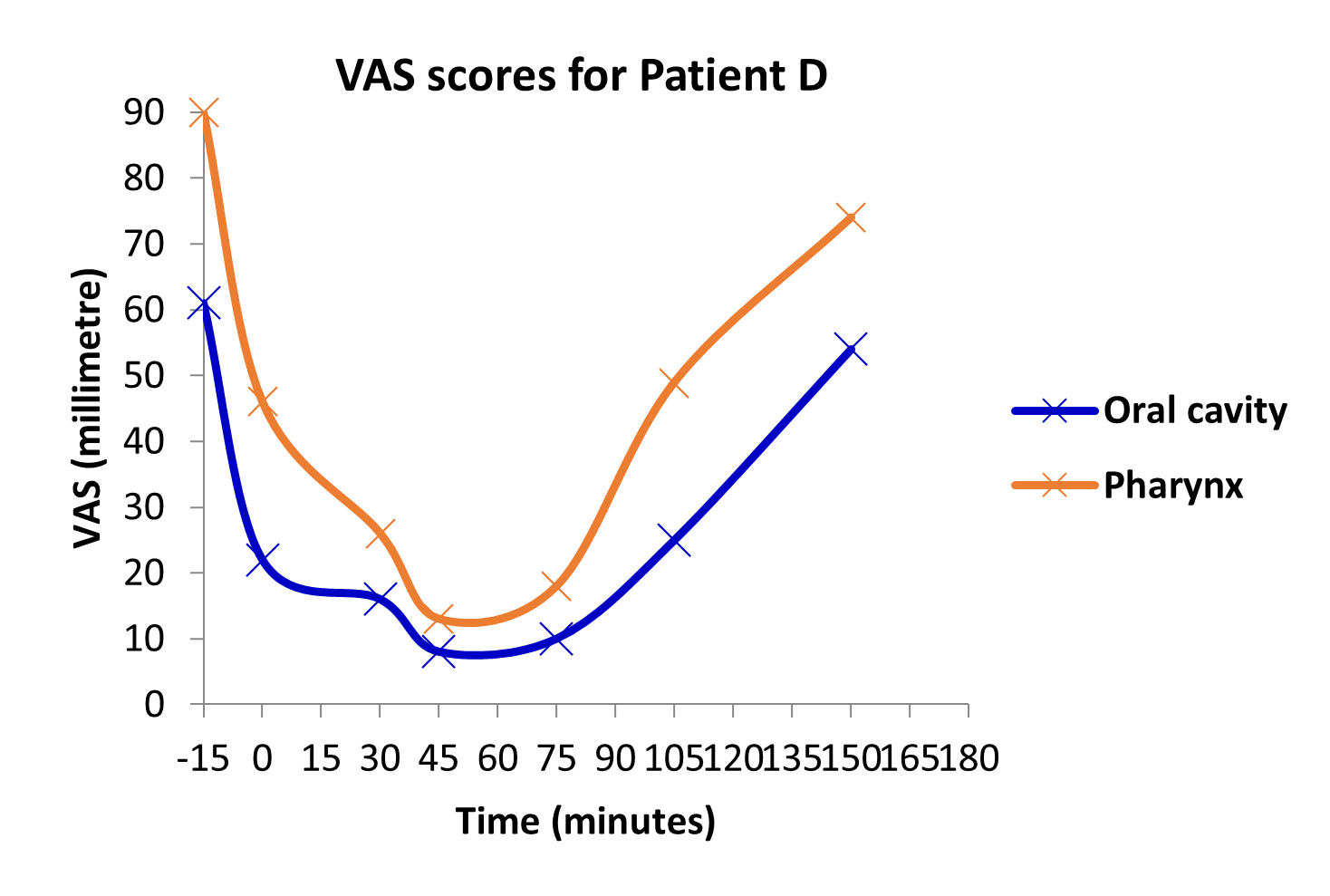
Lasting pain relief
Studies of BupiZenge™ has shown significant pain relief within minutes and lasting for hours.
Building on the well-known and researched active component bupivacaine, used for decades in surgery, dental procedures and epidural at child birth.
Oral
mucositis
Oral mucositis is a painful side effect from radiotherapy and chemotherapy. In som cancers nearly all patients develop oral mucositis. Different cancer types and chemotherapy treatments result in different severities 1-4 on WHO’s toxicity scale.
Agonizing pain
No escape
Patients experience varying degrees and severity of oral mucositis depending on cancer type and chemotherapy treatment. For some cancers like Head and Neck Cancer (HNC) 90% of all patients suffer from oral mucositis, with no good treatment options.
Quality of life
Debilitating pain has significantly negative impacts to quality of life. Studies have shown significant impacts to patients’ mental, physical and social well being due to the oral pain.
Affecting outcomes
Severe oral pain left untreated impact patients’ ability to eat, drink, sleep and socialize. Interrupted or reduced treatments and weight loss will impact cancer patients’ prognosis for recovery.
Potential to become the leading treatment for oral pain
OncoZenge holds a number of patents safeguarding
the innovation in and around our product application. There is a large commercial opportunity for OncoZenge and strategic partners in progressing our drug candidate to market.
Oral Mucositis
Mouth and throat surgery e.g. tonsillectomy
Dental applications
Endoscopy
Chronic pain (Burning Mouth Syndrome)
Dry mouth (Sjögren’s Syndrome)
Oral ulcerations (Behcet’s disease)
Oral inflammation (Oral Lichen planus)
Our lead indication is the condition oral mucositis, a market opportunity
in itself measuring hundreds of millions of dollars annually, even with conservative assumptions. The opportunity is global and an effective sales and distribution can reach millions of patients quickly considering the lack of good treatment options and the large unmet medical need.
Oral pain is a broader opportunity however, and a motivated partner may develop through incremental studies and approvals offer BupiZenge™ also for other uses. Such as general oral pain, endoscopy or dental applications, mouth and throat surgery such as tonsil removal, chronic pain such as Burning Mouth Syndrome (BMS) and more rare conditions such as Behcet’s disease or Oral Lichen Planus.
BupiZenge™ offers near term opportunities and long term growth potential.
Market research confirms the need for BupiZenge™
Bupivacaine safety data
Bupivacaine is a local anesthetic that is used to block pain signals from being sent to the brain. It is commonly used in dental procedures, surgery, and childbirth. Bupivacaine the maximum recommended daily human dose is (400 mg) This means that the total amount of bupivacaine that can be safely given to a patient in a 24-hour period is 400 mg. Our BupiZenge™ lozenge is 25 mg and recommended to give 5 times daily, ahead of meals.
Reference: https://www.accessdata.fda.gov/…./071165s020lbl.pdf
The systemic uptake of bupivacaine decreases with injection technique and injection time. This means that the amount of bupivacaine that enters the bloodstream is lower when it is injected slowly and with care.
Lozenges are a type of medication that dissolves slowly in the mouth. This means that the absorption of bupivacaine is slower when it is taken in lozenge form. This provides an additional margin of safety, as it means that less bupivacaine enters the bloodstream.
There is no risk of accidental (intravasal) high or/and fast exposure with lozenges that slowly dissolve. This is because the bupivacaine is released slowly into the bloodstream over time. If a lozenge is swallowed, the absorption of bupivacaine is even lower in the ventricle than in the mouth. This is because the pH in the ventricle is lower than in the mouth, which makes it more difficult for bupivacaine to be absorbed.

Research and publications
Read all the clinical studies and publications made on bupivacaines effects as a lozenge

